

Almost instantly, antibiotics were a miracle drug. But bacterial infections that can resist them are now one of the biggest global health threats—and it’s our fault. University of Rhode Island researchers take us inside the fight to save modern medicine as we know it.
By Nicole Maranhas
Perhaps you’ve heard the famous story. A scientist returns from his summer vacation to a messy lab and discovers that mold, blown in from an open window, has infiltrated a petri dish of disease-causing Staphylococcus and killed the bacteria. That’s the tale of Alexander Fleming and the birth of penicillin. A less-famous story: In 1945, the same year Fleming wins the Nobel Prize in medicine, he speaks at the Waldorf Astoria hotel at a dinner held in his honor and warns that “thoughtless” misusers of the drug will someday have blood on their hands.
He is quoted the next morning in The New York Times, in a column that runs on page 21 alongside news of meat and butter rations in restaurants, a tunnel to Staten Island, and Mighty Seventh war bonds. He cautions, “I hope this evil can be averted.”

To make this easy, there are catch phrases. “Cold or flu? Antibiotics don’t work for you.” “Snort. Sniffle. Sneeze. No antibiotics, please.” For Kerry LaPlante, stewardship comes down to the three Ds: “the right drug, in the right dose, for the right duration.”
LaPlante is a professor at the University of Rhode Island College of Pharmacy and director of the infectious disease research program at the Providence VA Medical Center. She is also co-director of the Providence VA’s Antimicrobial Stewardship Program, and vice chair of the Antimicrobial Stewardship and Environmental Cleaning Task Force for the Rhode Island Department of Health. Both are leading initiatives in the state to curb the overuse of antibiotics that drives antibiotic resistance, recently cited by the World Health Organization as one of our greatest public health threats. According to the U.S. Centers for Disease Control and Prevention (CDC), two million antimicrobial-resistant infections now occur each year (the term “antimicrobial” refers not only to antibiotics that treat bacterial infections, but also to drugs that treat infections caused by other microorganisms, such as fungi), resulting in 23,000 deaths. This number is expected to rise to 10 million deaths per year by 2050—in the U.S. alone. The European Centre for Disease Control and Prevention reports similar numbers overseas.
“Alexander Fleming warned us to be careful on overusing antibiotics,” says LaPlante. “We’ve only had them for 70 years, and here we are.”
Resistance, or the ability of disease-causing bacteria to fight off an antibiotic drug, isn’t anything new; the ongoing war between bacteria and the antibiotics found in nature, such as the one waged in Fleming’s petri dish, has been playing out for billions of years. But in recent years, bacterial infections that do not respond to antibiotic treatment have reached crisis levels.
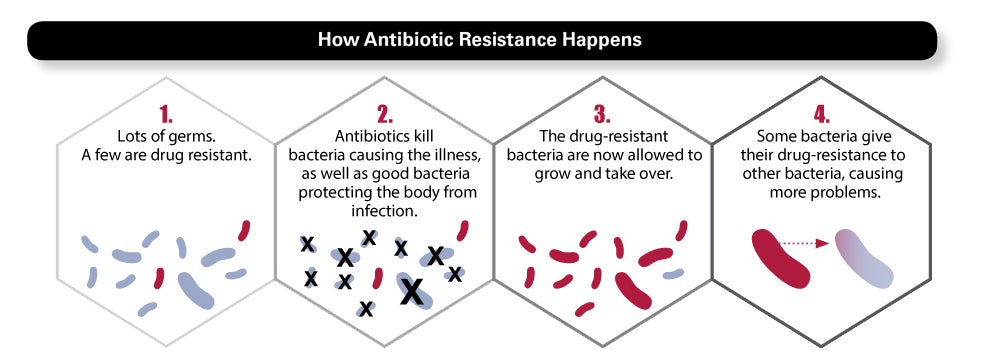
Here is what happens every time you take an antibiotic: While the drug kills most of the bacteria causing the infection, some bacteria will be naturally resistant. Meanwhile, the antibiotic also kills microflora in your gut, mouth, and skin—the good bacteria that protect you. As microflora are killed off, the “bad” bacteria have a window of opportunity to multiply, or spread their resistant genes to other bacteria. Often, our immune system catches them, but sometimes antibiotic resistant bacteria build big enough numbers to make trouble. Particularly if the immune system is weakened—a risk for patients in hospitals, nursing homes, and health care settings—these bacteria can cause a new infection, requiring a different antibiotic.
At the same time, we’ve raised the stakes. “If the body gets the wrong drug, dose, or duration, it can tease the bacteria into developing resistance,” says LaPlante. Skipping doses, sharing medications, saving leftover prescriptions to try to treat recurring infections (often caused by the very bacteria that don’t respond to the antibiotic you have already taken), or taking antibiotics when they aren’t needed—all these things can give healthy bacteria just enough exposure to train them to mutate and develop resistance to the drug.
In its 2015 report on the state of the world’s antibiotics, the Center for Disease Dynamics, Economics, and Policy showed a 30 percent increase in global antibiotics use between 2000 and 2010. As our use of antibiotics in health care and agriculture has grown, we have helped to launch armies of more powerful bacteria to fight them—exhausting our treatment options for a growing number of infections and outpacing our ability to discover new drugs.
Like any germ picked up on a door handle or on an airplane, drug-resistant bacteria spread easily from one person to another. This was ultimately Fleming’s warning and the crisis scientists are racing to solve.
Antibiotics can be challenging to prescribe. While a bacterial infection will respond to an antibiotic, infections caused by a virus will not. Newer technology, such as rapid diagnostic testing, can identify a bacterial infection within hours (many patients with strep throat, for example, can get same-day results), but otherwise, the cause of an infection may take days to diagnose. Rather than delay treatment in the meantime, prescribers may give patients a broad-spectrum antibiotic that can wipe out a range of possible infectious bacteria types—but broad-spectrum anti-biotics can also create a broad spectrum of resistance. “A lot of times, prescribers don’t know what is causing the infection, so they have to take their best guess at deciding if an antibiotic is necessary—and if it is the right drug, at the right dose, for the right duration,” says LaPlante. More widely-available rapid diagnostic testing could significantly help curb misuse and overuse.
Meanwhile, patients can also feel frustrated to leave their doctor’s office without a prescription. “The pressure is very apparent when you do primary care,” says David Dosa, a geriatrician at Rhode Island Hospital and an associate professor of medicine at Brown University, who has worked with LaPlante to help bring stewardship guidelines to nursing homes. “When people go to the doctor, they’ve reached a critical point where they’ve taken time off work and paid to see a doctor, and they want something that will help. If a doctor tells them it’s a virus and to go home, or to wait and come back, they are upset—and many doctors don’t want to upset their patients and risk losing them. Many will write a prescription so the patient feels heard.”
Data shows that 30 to 50 percent of antibiotics are prescribed unnecessarily—making stewardship programs critical in hospitals, long-term care facilities, nursing homes, and other health care settings. At the Providence VA, LaPlante works with a six-person team that includes two URI College of Pharmacy fellows, as well as Assistant Professor Aisling Caffrey, a pharmacoepidemiologist who helps LaPlante’s team track outcomes of antibiotic treatment. Each day, members of the team review a list of every antibiotic drug prescription in the hospital to ensure each patient receives the correct drug, dose, duration. In addition to being one of the most progressive stewardship programs in the state, it is also the only antimicrobial-resistance pharmacy training program in the country. “The program is a great partnership between doctors, pharmacists, nurses, and clinical microbiologists to protect patients,” says Providence VA’s Chief of Infectious Disease Melissa Gaitanis, who has co-directed the program with LaPlante since 2012.
LaPlante’s efforts have also included establishing clear-cut prescription guidelines (known as treatment pathways) for doctors. At URI, she works with College of Pharmacy Program Coordinator Jennifer DeAngelis to maintain an Antimicrobial Stewardship Program website, which has been a resource for physicians as far away as South Africa in eliminating the guesswork and error of antibiotics prescribing.

Drug-resistance is a problem humans created.
FALSE. Antibiotic-resistant bacteria will always exist. Overuse of antibiotic drugs, however, has helped create widespread infections that are resistant to multiple, or all, antibiotic treatments.
Antibiotics can treat viral infections, such as cold and flu.
FALSE. Colds and flu are caused by viruses. Antibiotics can fight only bacterial infections.
You don’t need antibiotics every time you have a bacterial infection.
TRUE. Doctors may prescribe a few days of “watchful waiting” before starting an antibiotic for mild bacterial infections that can get better without treatment.
I should stop taking an antibiotic when I feel better.
FALSE. Taking an antibiotic for the wrong duration (too long or too short a period) can be harmful. Talk with your prescriber about when to stop taking them.
If I develop a drug-resistant infection, I can spread it to another person.
TRUE. Bacteria, not people, become resistant to drugs. Bacteria spread easily through the air and on surfaces.

Do not take antibiotics for cold, flu, and other viral illnesses.
Take antibiotics as prescribed.
Never share medications or take an antibiotic prescribed to someone else.
Do not save leftover antibiotics to treat future infections.
Never pressure your doctor for an antibiotic.
Practice good hand-washing hygiene to prevent the spread of bacteria to yourself and others.
Ask your doctor about vaccines recommended to prevent infections.
Overall, stewardship is not only vital from a public health standpoint but from an economic one: The CDC estimates that the costs of antimicrobial resistance are as great as $35 billion per year in the U.S. alone. “Inappropriate use of antibiotics and health-care-associated infections are real threats to our national health-care infrastructure,” says Nicole Alexander-Scott, director of the Rhode Island Department of Health. “They also affect health-care expenditures in Rhode Island and could pose a threat to the stability of the health-care system in our state. Dr. LaPlante’s incredible passion, leadership, and expertise have been absolutely crucial to our work to address these issues.”
It was easy to call it a miracle drug.Penicillin cured the incurable—deadly infections from wounds, childbirth, strep throat. And as pharmaceutical companies rushed to discover and develop new anti-biotics to treat more types of infections, medicine leapt into a new era of possibilities, from organ transplants to chemotherapy, that were unthinkable before infections could be controlled. But miracles cannot be explained or understood—and understanding the way antibiotics work, as Fleming tried to warn, is critical.
In a recent World Health Organization survey of 10,000 respondents in 12 countries, 64 percent agreed that “Medical experts will solve the problem of antibiotic resistance before it becomes too serious.” So many health scares—SARS, bird flu, swine flu, mad cow disease—seem to stir up apocalyptic headlines, then vanish. To many of us, threats of a post-antibiotic era that sends us back to 1920s medicine are no different.
Fleming spoke multiple times of the need for antibiotic stewardship. Months after the Waldorf Astoria dinner, he gave his Nobel Lecture, delivering this hypothetical: Mr. X has a sore throat. He buys some penicillin and gives himself not enough to kill the streptococci but enough to educate them to resist penicillin. He then infects his wife. Mrs. X gets pneumonia and is treated with penicillin. As the streptococci are now resistant to penicillin, the treatment fails. Mrs. X dies. Who is primarily responsible for Mrs. X’s death? Why, Mr. X, whose negligent use of penicillin changed the nature of the microbe.
The crisis of antibiotic-resistant infections is complex, the collateral damage of drugs that every one of us, in some way, relies on. As we depend on scientists and medical professionals to solve it, they are also depending on us.

“Bacteria will always evolve to develop resistance to antibiotics.”

Meanwhile, the hunt is on. “We’ve been finding the same molecules over and over and over,” says David Rowley, URI’s chair of biomedical and pharmaceutical sciences. “If we want to find new drugs, we need to look in new places.”
The last new class of antibiotics was discovered decades ago. By the 1970s, most pharmaceutical companies turned away from developing new antibiotics—with a broad arsenal to treat infections and seemingly no more stones left unturned, they turned their attention to chronic disease.
By the time the looming antimicrobial resistance crisis grew unmistakably dire, developing new antibiotics had become a lengthy, expensive enterprise with almost no economic incentive and very little government support. In 2012, the Food and Drug Administration’s bipartisan GAIN Act (Generating Antibiotic Incentives Now) took a step toward stimulating drug companies to develop new medications.
Developing any new drug is a “long, arduous” path, says Rowley—from initial research and discovery, through pre-clinical and three phases of clinical trials, to FDA approval and post-approval monitoring, all of which can cost billions of dollars and take at least 10 years. But the first hurdle—finding new compounds that have never been tested or evaluated for antibiotic potential in the first place—is even more massive, sending scientists on a global scavenger hunt for clue-giving germs and never-before-seen compounds, searching everywhere from the scummiest subway stations to the blood samples of Komodo dragons.
And searching the ocean. “The marine environment offers a vast and relatively unexplored source of biodiversity for us to investigate,” says Rowley, whose research focuses on marine microbes with potential infection-fighting abilities. “A microbe that lives in the ocean has adapted to a different environment and is likely to have adapted different ways to kill pathogens.” One partner in his quest is David Smith, professor and associate dean of URI’s Graduate School of Oceanography, who has brought deep-sea sediment samples from his own research in locales as far-flung as the Arctic back to Rowley’s lab for testing—not only in pursuit of a potential antibiotic, but for a potential drug that could block bacteria from spreading or developing resistance at all. One of the most fascinating areas of Rowley’s research is quorum sensing, the language that bacteria use to coordinate their efforts. “If we can use other molecules to shut down the bacteria’s ability to communicate, we can prevent the bacteria from causing infection,” says Rowley. “Think of it this way. When we go to war, we usually take out the communication system of our enemy first.”
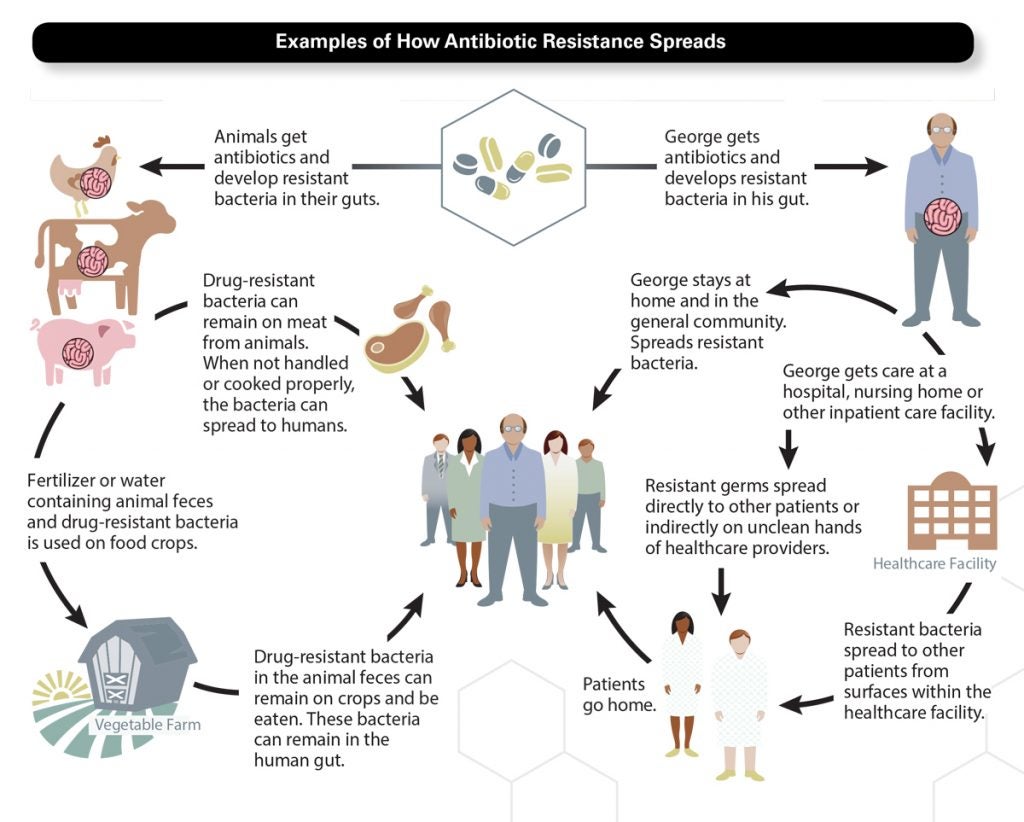
The enemy in this war is ingenious—bacteria have been successfully defeating antibiotics in nature for billions of years. Some use enzymes to break down antibiotic compounds. Some mutate, making themselves invasion-proof. Others can pump the drug out of their cells. Once bacteria acquire resistance, they multiply (each bacterium can divide into two in as few as 20 minutes) or transfer their resistant genes to healthy bacteria. The deadliest bacteria can build up an entire stockpile of warfare tactics. These show up on the news, with names like methicillin-resistant Staphylococcus aureus (MRSA), carbapenum-resistant enterobacteriacea (CRE), and Clostridium difficile (“C. diff.”), the “superbugs” that can withstand multiple, or all, antibiotics.
“Bacteria will always evolve to develop resistance to antibiotics,” says Rowley. “We need to stay one step ahead. I don’t know if we’ll ever have enough to overcome drug resistance. The more options we have available, the better we’re going to be.”
For Rowley, these options also include probiotics. Locally, he has worked with professors Marta Gomez-Chiarri and David Nelson in the College of Environment and Life Sciences, and also with shellfish farmers, to investigate how health-promoting bacteria can be used to prevent disease in aquaculture. That research could have further implications toward curbing antibiotics in agriculture generally—a critical task, given that agricultural demands are projected to rise to 105,600 tons, or nearly two-thirds of the world’s total antibiotic applications, by 2030. “If we can find beneficial microbes that can prevent disease in plants and animals, we can avoid the use of antibiotics in food consumption,” says Rowley.
He adds, “This is all hands on deck. There isn’t one solution to this problem.”
Hands. Eighty years before penicillin changed the world, the power of hand washing to prevent infection was a revolutionary discovery. It doesn’t sound exciting now, but it did when Hungarian physician Ignaz Semmelweis realized that this simple act nearly eliminated deaths from puerperal fever in the maternity clinic at the hospital where he worked.
After using the bathroom, while preparing food, before eating, before and after changing diapers, or when caring for someone who is sick, it takes 20 seconds to prevent the spread of germs and potentially save lives. Fittingly, that’s two rounds of “Happy Birthday,” as the rule of thumb goes—one for others, and one for yourself.
As Rowley notes, there won’t be one solution to the threat of drug-resistant infections. Science may find the next miracle, but prevention and stewardship will always be key. Taking antibiotic drugs only when needed and as directed, raising awareness, keeping hands clean. Whatever miracles await, you are one of the heroes in this story. •
 Home
Home Browse
Browse Close
Close Events
Events Maps
Maps Email
Email Brightspace
Brightspace eCampus
eCampus



