uri physics colloquium
Date and Time : Monday, July 1st 2019 at 11 am.
Location : East Hall Room 112.
Modeling pH effects at the water/membrane interface: application to the pHLIP peptide
Miguel Machuqueiro, Universidade de Lisboa
abstract
CQB & BioISI, Departamento de Química e Bioquímica, Faculdade de Ciências, Universidade de Lisboa, 1749-016 Lisboa, Portugal
pH is a crucial physicochemical property that affects most biomolecules. Changes in protonation equilibrium of susceptible sites will modify the electrostatic environment and, consequently, have an effect on peptide/protein structure, stability and function.1 The pKa values of common titrable sites can be significantly 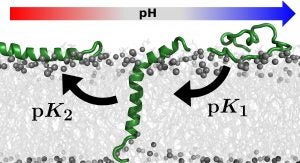
influenced by changes in solvent mixture, by direct interaction with other proteins or due to insertion in a lipid bilayer.2
The pH (low) insertion peptides (pHLIPs) is a family of peptides that are able to insert into cell membranes with an acidic vicinity,3 such as tumor cells, thus working as an efficient tumor-specific biomarker or drug carrier.4 The molecular mechanism of pHLIPs insertion, folding, and stability in the lipid bilayer at low pH is based on multiple protonation events, which are challenging to study experimentally at the molecular level.
Constant-pH molecular dynamics (CpHMD) methods have been used successfully to sample protonation behavior of titrable amino acids inserted into a lipid bilayer.2,5 Here, we use this computational approach to help identify the role of key protonable residues in the membrane stability of pHLIP.5 Also, by proposing peptide sequences with new functions (see Figure), we may help strengthening the technological application of pHLIPs as biomarkers and possible drug-delivery systems for tumor cells.
 URI Homepage
URI Homepage









