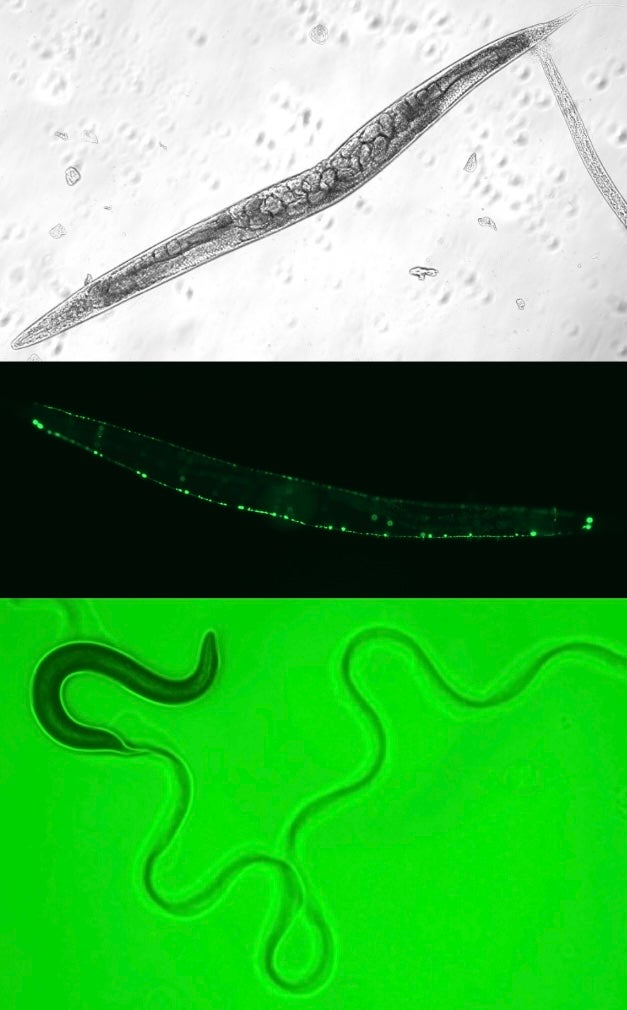
Although FA is primarily characterized by increased risk for bone marrow failure and cancer, several recent clinical reports describe pleiotropic neurological symptoms in FA patients, including abnormal brain MRIs, seizures, brain lesions, and early-onset cognitive decline. This constellation of neurological symptoms is collectively referred to as Fanconi Anemia Neurological Syndrome (FANS). The molecular etiology of FANS is unknown. Recent omics data from our lab and others suggests that the FANCD2 protein may play an important role in nervous system development under conditions of replication stress. For example, ChIP-seq analysis has uncovered that the FANCD2 protein binds to several transcriptionally active large neural genes upon treatment with the DNA polymerase inhibitor aphidicolin. Many of these large neural genes are linked to neuropsychiatric and neurodevelopmental disorders. Gene set enrichment analysis (GSEA) of RNA-seq data from FA-D2 (FANCD2-/-) and FANCD2-complemented patient cells has also uncovered differential regulation of neurogenesis and retinoic acid metabolism pathways in FA-D2 (FANCD2-/-) cells. We are currently using the model nematode Caenorhabditis elegans to study the role of the worm ortholog of FANCD2, FCD-2, in nervous system development. C. elegans has an exceptionally well-characterized nervous system with exactly 302 neurons, making it an excellent model for neuronal studies. conditions of replication stress. We anticipate that our findings will yield much needed insight into the origins of FANS and open new avenues of potential therapeutic intervention.