Motion: On
Motion: Off
Contrast: Standard
Contrast: High
Apply site-wide
The PDI enables partner firms to rapidly and cost-effectively achieve stable formulations that can be readily transferred to a GMP contract manufacturer. We also provide analytical development services for all dosage forms, which may include formulations developed in-house or samples provided by partner firms.
PDI Facilities
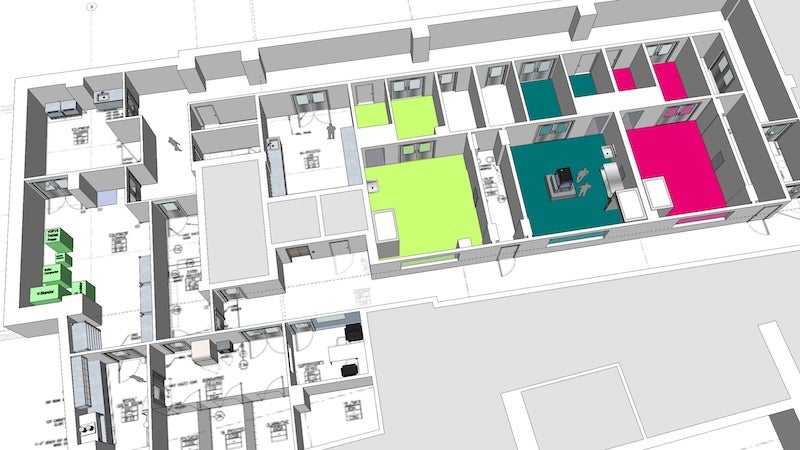
- 6,000 sq. ft. manufacturing* facility, including three independent process suites
- 1,500 sq. ft. analytical development and stability testing laboratory
Contract Development and Manufacturing*
- The PDI provides formulation/process development and manufacturing* of solid oral dosage forms (e.g., tablets, capsules, powders, etc.).
- Our equipment and capabilities help our client firms achieve product quality expectations and meet clinical development milestones with adequate supplies for development and stability studies.
*non-GMP manufacturing
