Submitted by Heidi Pickard, PhD Candidate at Harvard University, STEEP Trainee

Fish consumption advisories are a non-enforceable, public health tool that aim to reduce chemical exposures to consumers via recommended consumption limits of fish based on the levels and toxicity of the detected chemicals. Current regulatory efforts focus on a few PFAS chains, such as PFOS, which have been around for decades and are known to accumulate in seafood; regulation does not consider the broad range of other PFAS that may also be present.
PFAS precursors are chemical structures that can break down in the environment, creating resultant PFAS that are extremely persistent once in their final form. However, these precursors can also persist for a long time and accumulate in seafood before breaking down. We know very little about toxicity and effects, and thus, fish consumption risk evaluations should consider potential human exposure to the full suite of PFAS and precursors.
It is challenging to measure many of these other PFAS, limiting our ability to account for them in risk evaluations. To determine the concentration of a chemical in a seafood sample, we first need to extract that chemical from the sample. We then identify that chemical using an instrumental technique known as liquid chromatography-tandem mass spectrometry (LC-MS/MS), which tells us if that chemical is present in our sample. To quantify how much of the chemical is in our sample after identifying it, we need a standard of that chemical to compare to our sample. Because PFAS are a massive group of thousands of chemicals, it is nearly impossible to measure for all PFAS in a sample. Instead, we are limited to only being able to quantify around 40 PFAS based on the number of standards we have available to us, which is called targeted analysis. This can lead to underestimations in how many PFAS are accumulating in our seafood and by proxy, to how much we’re really exposed.
To combat this limitation, we use additional methods in our lab (Figure 1) to help us identify other PFAS that might be present in a sample. One method involves determining the amount of organofluorine present in a sample. Because PFAS are organic fluorine compounds, meaning that they have a carbon-fluorine bond, we can extract all the organofluorine present in a sample, called extractable organofluorine (EOF), and measure how much of that is present using an instrument known as combustion ion chromatography (CIC). If we compare the amount of EOF to the 40 PFAS we measured by targeted analysis, then we can determine how much organofluorine could be coming from other unknown PFAS such as those PFAS precursors.
To further examine what the unknown PFAS precursors might be, we use another method, called total oxidizable precursor (TOP) assay, which oxidizes the sample, breaking down precursors into the other PFAS that can be measured. Once we break down the precursors and measure the oxidized sample on the LC-MS/MS instrument, we can analyze results to identify unknown precursors and quantify levels. If we still haven’t identified all the PFAS in our sample, we can also measure the sample with an instrument that allows us to identify additional PFAS based on their chemical structure, limited in that we cannot quantify concentrations without matching standards. This process is called non-targeted analysis. Combining methods allows us to gain a broader picture of the extent of PFAS contamination present in our seafood samples.
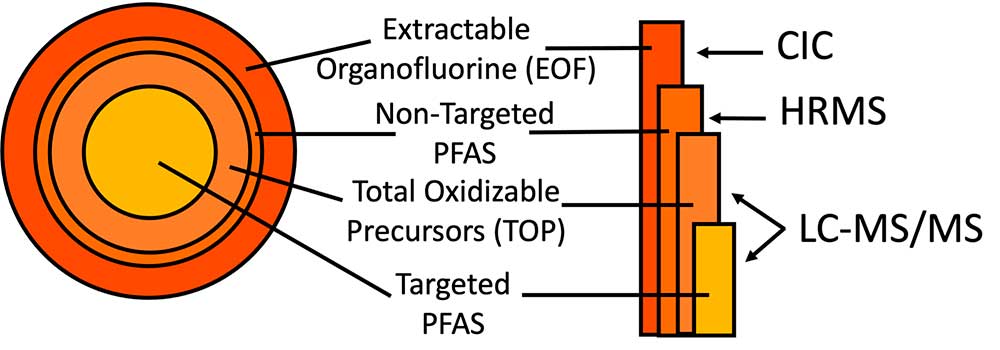
Using the toolbox of methods, we measured PFAS in muscle tissues from eight species of freshwater fish commonly caught by recreational fishers from nine surface water bodies in southern New Hampshire, U.S. We found PFOS to be the most abundant compound with the highest concentrations present in the various fish species. We also found that the major PFAS measured in the water were different than the major PFAS measured in the fish (Figure 2), as result of differing chemical properties between PFAS, like structure and size. PFAS with a longer chain of carbon-fluorine bonds are more likely to accumulate in fish, whereas PFAS with less carbon-fluorine bonds usually stay in the water. Using combined methods, we also found that certain PFAS precursors, particularly those with a shorter carbon-fluorine chain and sulfonamide in the chemical structure, also accumulated in the fish. This is a critical finding and has not yet been commonly reported due to challenges with measuring these PFAS. Further results of our research can be found in the journal of Environmental Science & Technology publication.
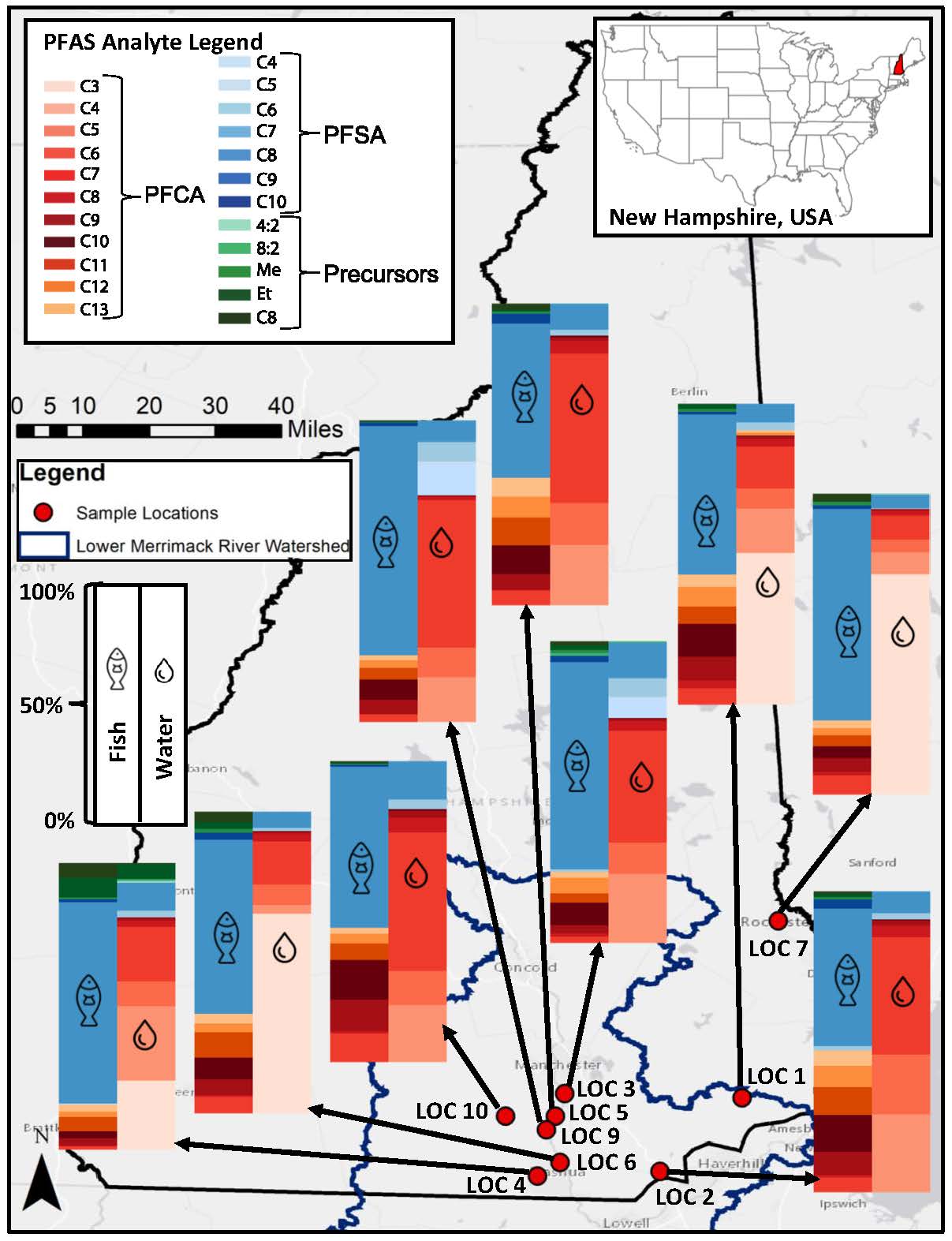
Through this work, we were able to gain valuable insights into the accumulation of PFAS precursors in freshwater food webs. We found that because fish accumulate distinct chemicals not similarly retained in the surrounding environment, water monitoring data is not always useful for regulating fish consumption. Additionally, physicochemical and toxicological data for many of these PFAS precursors are currently lacking, making it difficult to assess potential risks from exposure to these compounds. Moving forward, we recommend the development of analytical standards for these bioaccumulative precursors so that their concentrations can be accurately quantified in samples, and so that toxicity assessments can be conducted, improving the accuracy of advisories for consumers in the future.
Heidi M. Pickard, Bridger J. Ruyle, Colin P. Thackray, Adela Chovancova, Clifton Dassuncao, Jitka Becanova, Simon Vojta, Rainer Lohmann, and Elsie M. Sunderland. PFAS and Precursor Bioaccumulation in Freshwater Recreational Fish: Implications for Fish Advisories. Environmental Science & Technology 2022 56 (22), 15573-15583. DOI: https://doi.org/10.1021/acs.est.2c03734

