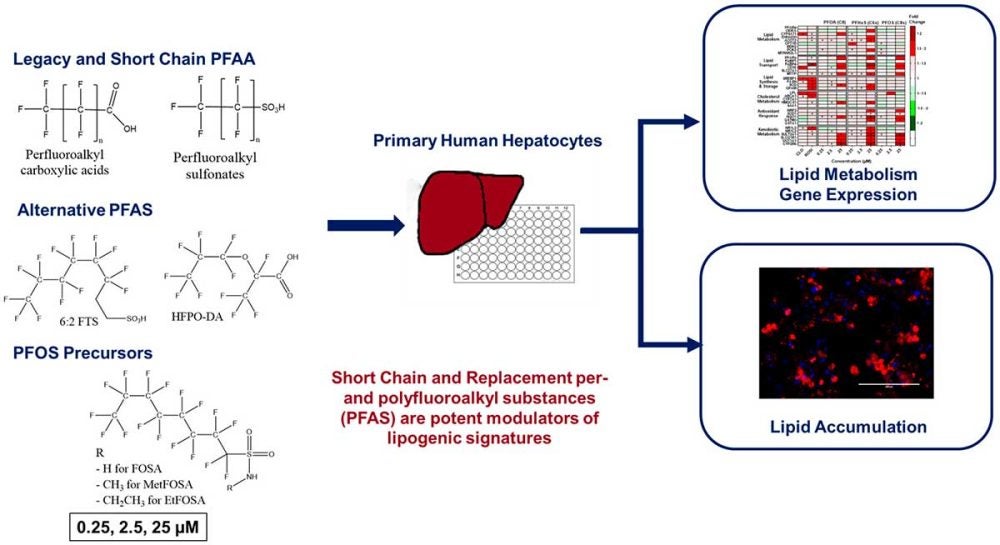
Former STEEP trainee Emily Marques and Dr. Angela Slitt of the Department of Biomedical and Pharmaceutical Sciences at the University of Rhode Island worked with project collaborators to investigate the biological ramifications of legacy and alternative per- and polyfluoroalkyl substances (PFAS) exposure on human liver cells, as described in their May 2022 publication in Toxicology and Applied Pharmacology.
Shifts in the manufacturing industry to produce alternative and “short-chain” PFAS chemicals compounds were aimed at decreased bioaccumulation and environmental persistence. These alternatives are prevalent in PFAS production after industry phase-outs of legacy PFAS chemicals such as perfluorooctanoic acid (PFOA) and perfluorooctanesulfonate (PFOS). Still, scientists continue to find alternative and short-chain compounds present in wildlife, humans, and throughout the environment. Current knowledge on PFAS exposure in humans and model organisms recognizes adverse health effects of chemical exposure linked to immune disruption, kidney cancer, and biomarkers of liver injury.
Marques, Slitt, and collaborators aimed to explore the effects of PFAS exposure on gene expression in a group of human liver cells known as hepatocytes. Specifically, they were interested in determining if PFAS exposures affected genes linked to hepatic steatosis, or fatty liver disease. The study exposed cryopreserved human hepatocytes to varying concentrations of individual PFAS compounds and employing biopharmaceutical techniques to quantify gene expression and observe changes in structural morphology of hepatocytes.
Results showed that legacy PFAS compounds such as PFOA, perfluorohexanesulfonate (PFHxS), and PFOS induced, at a minimum, 7 of 35 gene transcripts directly linked to lipid metabolism, lipid transport, and antioxidant response pathways when hepatocytes were exposed at the highest concentration of individual compounds in experimental treatment groups. The legacy compounds also induced gene expression related to xenobiotic metabolism – i.e. Cytochrome P450 2B6 and Sulfotransferase 2A1 – when hepatocytes were exposed to the highest experimental concentrations.
A key finding was the capacity for shorter chain PFAS and fluorinated alternatives to induce significant liver lipid accumulation and gene activation at lower exposure concentrations than legacy PFAS, providing substantive evidence that fluorinated alternatives show potential to behave as potent gene inducers and should be further investigated for impacts to humans.
Read the full article: “Replacement per- and polyfluoroalkyl substances (PFAS) are potent modulators of lipogenic and drug metabolizing gene expression signatures in primary human hepatocytes,” Marques, E., et. al.