Aromatic amines are among the most notorious environmental chemicals. At pathologically relevant concentrations, their reactive metabolites can react with cellular DNA to produce bulky lesions. If not efficiently repaired, these lesions can result in mutations that lead to various sporadic cancers. Understanding the mechanisms by which these lesions are recognized by the XPC nucleotide excision repair complex is the focus of this proposal.
Project Summary
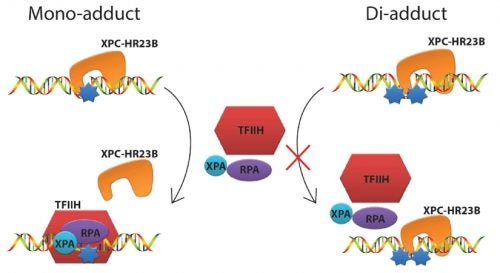
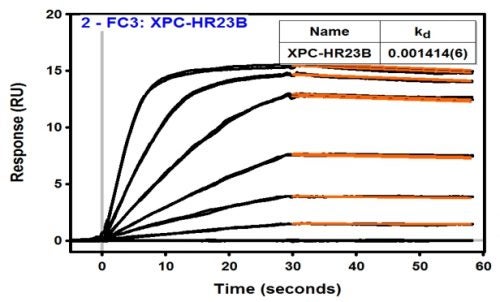
The human genome is under constant assault by environmental factors that include sunlight and chemicals. Arylamines are diverse and ubiquitous environmental mutagens implicated in the etiology of various sporadic cancers. Direct exposure to arylamines, such as 4-aminobiphenyl (ABP), increases the risk of bladder cancer, the fourth most common cancer in men in the US. Arylamines absorbed into the cells are activated and react with cellular DNA to produce bulky DNA adducts. If not efficiently repaired, these lesions can result in mutations and tumorigenesis. Nucleotide excision repair (NER) is a key repair pathway that protects the integrity of the genome against diverse DNA lesions including arylamine adducts. To initiate NER, the xeroderma pigmentosum C (XPC) protein complex first detects the lesions and recruits downstream factors, which in turn verify, excise and restore the damaged portion of the DNA. Though it has been generally believed that stable, specific binding to XPC is required for efficient repair, we recently found that it is not always true: for certain arylamine lesions in a highly mutagenic sequence context, an extremely tight binding to XPC (due to a slow dissociation rate) may, in fact, hamper NER (Hilton et al., PLos One e0157784 (2016)). In this project we will collaborate with Dr. Junghyun Min at Baylor University to investigate the structural and mechanistic basis underlying this intriguing relationship between the XPC-binding and repair potentials of arylamine lesions. We will use a powerful multidisciplinary approach that combines the complementary strengths of 19F-NMR, surface plasmon resonance (SPR), and X-ray crystallography. The knowledge gained on how certain lesions evade repair while tightly binding to repair proteins, may also lead to novel ideas and agents to combat cancer.