PFAS compounds have been manufactured for decades and applied to a wide range of applications. While PFAS introduction into the environment has occurred with relative ease, developing methods for detecting, measuring, and removing these forever chemicals is daunting. Fortunately, research and remediation utilize similar methods, so optimization of sampling strategies is key, and the materials for collecting samples must have high affinity for the desired PFAS and minimal collection of undesirable molecules. Material effectiveness should not be hindered by organic matter or ions, and the ability to regenerate the material for future use is cost-effective. One common material for sampling PFAS is adsorbent resin. Adsorbents, or sorbents, produced from activated carbon, silica gel, aluminum, etc., bind to and retain molecules. The structure of adsorbent resins are porous, spherical beads, and can be engineered to target certain types of PFAS.
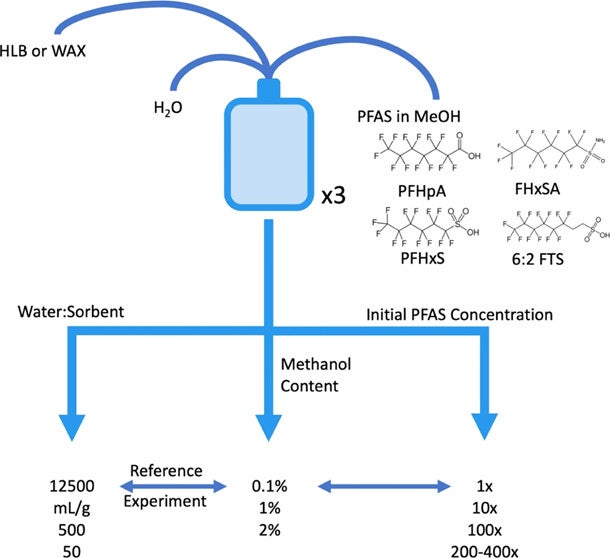
Research compared the effectiveness of two commonly used types of PFAS sorbents (hydrophilic-lipophilic balance [Oasis HLB]; weak anion exchange [Oasis WAX]) to determine which was best suited for PFAS sampling, and additionally how to optimize research and minimize error during this type of testing.
Researchers found that the variation in experimental results was highly sensitive to changes in the experimental set-up. High analytical uncertainties occurred when the ratio of sorbent to environmental sample solution or initial concentration of PFAS were too high. Addition of methanol is necessary in most PFAS spiking solutions (PFAS are more stable in methanol), but the presence of methanol in samples can affect the calculation of key coefficients that are needed to determine sorbent effectiveness. Careful consideration of experimental methods will support optimization of these laboratory experiments, and contribute to best practices of collection and remediation of PFAS.
Snook, J., Becanova, J., Vojta, S., Lohmann, R. 2023. Avoiding Artifacts in the Determination of Per- and Polyfluoroalkyl Substance Sorbent−Water Distribution. ACS EST Water, 3, 2355-2362. DOI: https://doi.org/10.1021/acsestwater.3c00084