URI Pharmaceutical Development Institute offers suite of online, in-person courses, launching online Training Center
The University of Rhode Island Pharmaceutical Development Institute is revolutionizing pharmaceutical training and workforce development opportunities in biopharmaceutical processing and manufacturing, helping meet the critical demand for skilled professionals. The PDI continues to provide solutions for training even in these challenging times, offering live online and in-person courses throughout the COVID-19 pandemic.
The Institute launched its online Training Center Dec. 16, offering easy access for pharmaceutical firms to train their staffs even during the pandemic. The Institute’s combination of deep industry knowledge and educational expertise ensures the delivery of industry-focused value to all participants. Pharmaceutical employees working already in the field, as well as students just beginning their careers, get the education they need through the PDI’s comprehensive scheduled training sessions, and its dynamic customized programs tailored to a contracting company’s specific needs.
Current PDI scheduled course offerings include:
 Intro to Biopharmaceutical Manufacturing. This course is designed for biopharmaceutical manufacturers to ensure employees achieve proficiencies in both upstream and downstream biopharmaceutical manufacturing. It begins with the foundational theories of biopharmaceutical processing, then move into the methodologies and principles of mammalian cell culture and aseptic upstream operations. Students learn the importance of aseptic techniques, and apply this knowledge in our hands-on laboratories.
Intro to Biopharmaceutical Manufacturing. This course is designed for biopharmaceutical manufacturers to ensure employees achieve proficiencies in both upstream and downstream biopharmaceutical manufacturing. It begins with the foundational theories of biopharmaceutical processing, then move into the methodologies and principles of mammalian cell culture and aseptic upstream operations. Students learn the importance of aseptic techniques, and apply this knowledge in our hands-on laboratories.
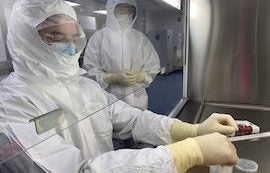 Upstream Aseptic Processing. This course is designed for biopharmaceutical manufacturers to ensure that their employees, who are working in upstream processing, have clear understandings of and technical acumen for aseptic cell culture and scale up. Lectures and hands-on laboratories prepare participants to work effectively in upstream bioprocessing suites which includes maintenance and monitoring of aseptic environments while culturing mammalian cells.
Upstream Aseptic Processing. This course is designed for biopharmaceutical manufacturers to ensure that their employees, who are working in upstream processing, have clear understandings of and technical acumen for aseptic cell culture and scale up. Lectures and hands-on laboratories prepare participants to work effectively in upstream bioprocessing suites which includes maintenance and monitoring of aseptic environments while culturing mammalian cells.
 Advanced Chromatography. This course is intended to educate participants in the principles and practices of current chromatography methods used for advanced downstream refinement of therapeutic proteins in the biopharmaceutical industry. Theories and best practices, critical for success in industrial chromatography, are presented including resin and column choices, as well as column packing and final protein purification.
Advanced Chromatography. This course is intended to educate participants in the principles and practices of current chromatography methods used for advanced downstream refinement of therapeutic proteins in the biopharmaceutical industry. Theories and best practices, critical for success in industrial chromatography, are presented including resin and column choices, as well as column packing and final protein purification.
 Facilities Cleaning & Environmental Monitoring. This course introduces biopharmaceutical personnel to regulatory expectations and industry trends in the disciplines of facility cleaning, validation and environmental monitoring (EM). Instruction and hands-on laboratory work focus on essential industry guidance protocols that govern the effectiveness of cleaning procedures in a certified clean room.
Facilities Cleaning & Environmental Monitoring. This course introduces biopharmaceutical personnel to regulatory expectations and industry trends in the disciplines of facility cleaning, validation and environmental monitoring (EM). Instruction and hands-on laboratory work focus on essential industry guidance protocols that govern the effectiveness of cleaning procedures in a certified clean room.
Upcoming courses:
- Validation Concepts in Biopharmaceutical Manufacturing. Participants attain knowledge of the key elements of validation, including regulatory guidance.
- Cell Harvesting. Students learn to apply the theories and practices of mammalian cell harvest and separation protocols through a comprehensive lecture and hands-on lab.
The URI Pharmaceutical Development Institute is affiliated with the URI College of Pharmacy and housed in Avedisian Hall on the Kingston campus.

