CRCF Instrumentation in Action!
The collaboration between George and Anne Ryan Institute for Neuroscience at the University of Rhode Island (URI) and the Feinberg School of Medicine at Northwestern University aimed to investigate neuroplasticity in amyotrophic lateral sclerosis (ALS), utilizing sophisticated instrumentation at the CRCF, and resulted in the publication “Inhibitory interneurons show early dysfunction in a SOD1 mouse model of amyotrophic lateral sclerosis” in the Journal of Physiology (February 2023. PMID36515374. PMCID9898203). RI-INBRE supported co-authors: Kayleigh J. LaPre (SURF ’19), and Assistant Professors of URI Biomedical and Pharmaceutical Sciences/Ryan institute for Neuroscience Dr. Marin Manuel and Dr. Katharina A. Quinlan (URI SURF Mentors).
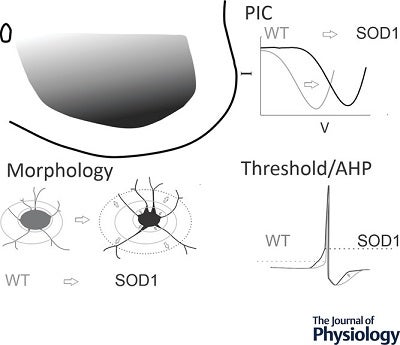
This published study investigated the role of inhibitory interneurons in ALSusing a SOD1 mouse model, specifically focused on glycinergic interneurons in the ventral lumbar region. The excitability and morphology of these interneurons were directly assessed during the early postnatal stage. The patch clamp technique was used to measure excitability, revealing decreased excitability in SOD1 interneurons. They exhibited depolarized persistent inward currents (PICs), increased voltage and current thresholds for firing action potentials, and a slight decrease in afterhyperpolarization duration. Morphological analysis showed that the primary neurites of ventral SOD1 inhibitory interneurons were larger in volume and surface area compared to wild-type mice.
The study analyzed over 2000 interneurons, including approximately 1000 from both wild-type and SOD1 mice. The CRCF Nikon Eclipse Ti2 inverted confocal microscope captured Z-stack photomicrographs of glycinergic interneurons in the lumbar ventral horn at ×20 magnification and Neurolucida software produced three-dimensional reconstruction of GlyT2-positive interneurons. The research revealed inhibitory interneurons in the SOD1 mice exhibited decreased excitability, including depolarized persistent inward currents (PICs) and increased threshold for firing action potentials. Also, primary neurites of the interneurons were larger in volume and surface area compared to wild-type mice.
The researchers further categorized the GlyT2 interneurons based on their location within the ventral spinal cord. The interneurons near the ventral white matter, where Renshaw cells are located, were most significantly affected, showing more depolarized PICs and larger primary neurites. Interneurons in lamina IX exhibited depolarized PIC onset, while those in laminae VII and VIII were least affected.
Overall, the study indicates that inhibitory interneurons in ALS exhibit early region-specific dysfunction, which could disrupt the balance between excitation and inhibition of motoneurons, modify motor output. The authors also raise concerns that therapeutics like riluzole, which reduce central nervous system excitability, could exacerbate the inhibitory dysfunction observed in this study.
The utilization of cell membrane-coated nanoparticles in the delivery of therapeutics
A collaboration between researchers from the University of Rhode Island (URI) and 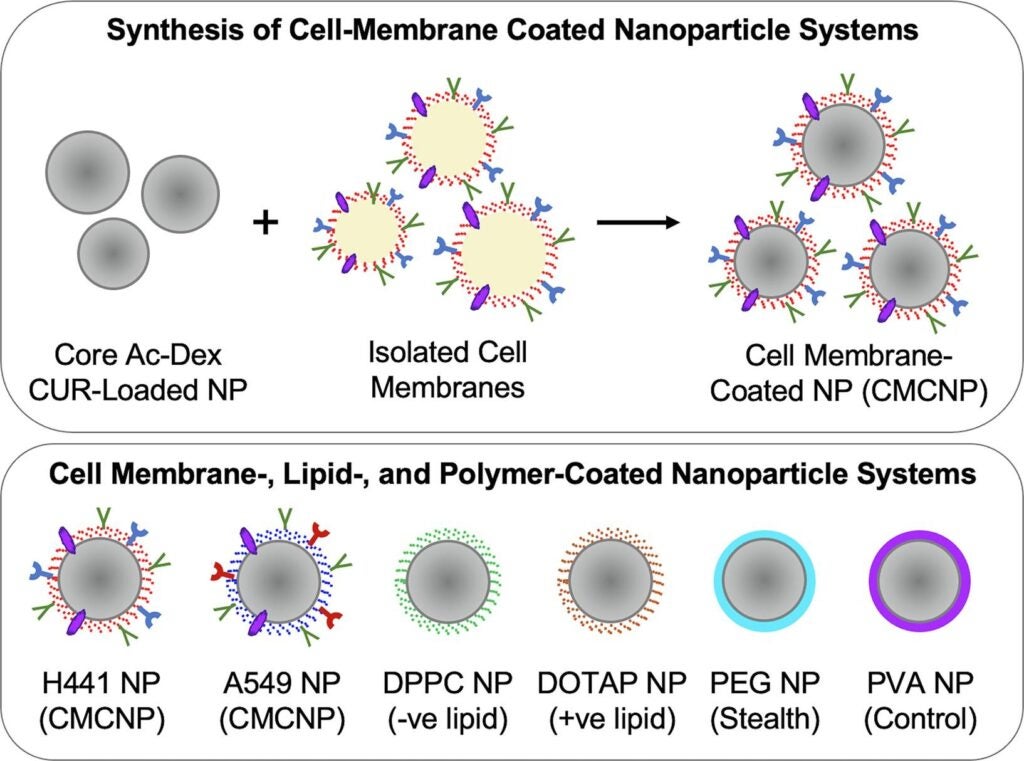 Brown University resulted in the creation of cell membrane-coated nanoparticles (CMCNP), which involve coating a core nanoparticle (NP) with cell membranes. The CMCNP have been gaining attention due to their ability to mimic the properties of the cells, allowing for enhanced delivery and efficacy of therapeutics.
Brown University resulted in the creation of cell membrane-coated nanoparticles (CMCNP), which involve coating a core nanoparticle (NP) with cell membranes. The CMCNP have been gaining attention due to their ability to mimic the properties of the cells, allowing for enhanced delivery and efficacy of therapeutics.
Md Golam Jakaria a former graduate student from URI’s Department of Chemical Engineering, published data in a CMCNP-related paper with Dr. Samantha Meenach, by using several instruments at RI-INBRE’s Centralized Research Core Facility (CRCF) in 2022.
The research at the CRCF utilized a combination of biophysical-, nuclear magnetic resonance (NMR)-, confocal microscopy- and biochemical- experiments to evaluate the internalization and translocation of CMCNP into and across pulmonary epithelial cells in vitro.
The Nikon Eclipse Ti2 Inverted Confocal Microscope, located at CRCF, provided comprehensive 3-D information about the cellular specimens by high-precision Z-axis readout. The confirmed internalization of NP into H441 cells was supported by data from the confocal imaging experiments and utilized to evaluate fluorescence quantification of NP internalization with respect to the number of cells. The Bruker 300 MHz NMR from CRCF provided the analysis of the polymer, acetalated dextran (Ac-Dex). The cyclic-to-acyclic (CAC) acetal ratio and the total amount acetal coverage was confirmed by 1H NMR spectroscopy. Ac-Dex, utilized as the biodegradable polymer in the core NP, is an acid-sensitive, biodegradable, dextran-based polymer, extensively used in drug delivery applications.
Additional instrumentation from the CRCF included ultracentrifuges in the cell membrane-coated nanoparticle preparation.
Md Golam Jakaria, Parand Sorkhdini, Dongqin Yang, Yang Zhou, and Samantha Meenach published “Lung cell membrane-coated nanoparticles capable of enhanced internalization and translocation in pulmonary epithelial cells” in the International Journal of Pharmaceutics. February 5, 2022. PMID34954003 PMC8792290
Complete Genome of Aeromonas encheleia Strain SOD01 Isolated from an Urban Freshwater Stream
Dr. Laura E. Williams’ undergraduate research group at Providence College sequenced the complete genome of Aeromonas encheleia utilizing the CRCF/MIC’s Sequencing Center, where they isolated SOD01 DNA from the organism found in a freshwater stream in Rhode Island. Dr. Williams, Associate Professor of Biology said, “In addition to my research lab, I’ve also used data generated by the sequencing center in my Genomics class.”
Williams’ areas of expertise include Genomics, bioinformatics, symbiosis, molecular evolution, bacterial lifestyle, and antibiotic resistance. Her lab has two research tracks: predatory bacteria and the microbiome. Dr. Williams said, “We have isolated and sequenced predatory bacteria from different environments throughout Rhode Island and the surrounding area. We are using comparative genomics to examine the evolution of different gene families in these predatory bacteria.” Her group is also assaying predatory phenotypes such as prey range and predation efficiency to determine variation in the outcomes of interactions between predatory bacteria and prey. An assay is a laboratory test to locate and measure the quantity of a specific substance.
Their research “Complete Genome of Aeromonas encheleia Strain SOD01 Isolated from an Urban Freshwater Stream” was published in Microbiology Resource Announcements in September 2022. PMID35980180 PMC9476934
Surface Plasmon Resonance (SPR) for COVID-19 Research
 COVID-19 research utilizing the Centralized Research Core Facility’s (CRCF) instrumentation, Biacore T200 Surface Plasmon Resonance (SPR), made the cover of the Journal of Agricultural and Food Chemistry’s October 2021 issue (PMID34586788, PMC8491554). The manuscript, “Inhibitory Effects and Surface Plasmon Resonance-Based Binding Affinities of Dietary Hydrolyzable Tannins and Their Gut Microbial Metabolites on SARS-CoV-2 Main Protease” resulted from a significant collaboration between the Seeram and Cho labs at the University of Rhode Island. Their findings may provide insights into understanding the effects of tannins and their metabolites on SARS-CoV-2 Mpro.
COVID-19 research utilizing the Centralized Research Core Facility’s (CRCF) instrumentation, Biacore T200 Surface Plasmon Resonance (SPR), made the cover of the Journal of Agricultural and Food Chemistry’s October 2021 issue (PMID34586788, PMC8491554). The manuscript, “Inhibitory Effects and Surface Plasmon Resonance-Based Binding Affinities of Dietary Hydrolyzable Tannins and Their Gut Microbial Metabolites on SARS-CoV-2 Main Protease” resulted from a significant collaboration between the Seeram and Cho labs at the University of Rhode Island. Their findings may provide insights into understanding the effects of tannins and their metabolites on SARS-CoV-2 Mpro.
The collaboration utilized a combination of biochemical-, SPR-, and docking-based assays to evaluate the inhibition and binding affinities of a series of tannins and their gut microbial metabolites, on severe acute respiratory syndrome coronavirus (SARS-CoV-2) main protease (Mpro). SARS-CoV-2 Mpro is a non-structural protein that breaks down the viral polyproteins to generate other non-structural proteins, namely RNA-dependent RNA polymerase and the helicase essential for viral replication.
The CRCF Biacore T200 was used to measure the direct binding affinities of tannins and their gut microbial metabolites with SARS-CoV-2 Mpro. The SPR binding assays revealed that hydrolyzable tannins and the PYG metabolite can directly bind to the SARS-CoV-2 Mpro protein, but the druggability seems low.
The CRCF’s SpectraMax M2 plate reader was applied for the SARS-CoV-2 Mpro enzyme inhibition assay. Dietary hydrolyzable tannins exerted promising inhibitory effects on SARS-CoV-2 Mpro activity at high concentrations (10 and 50 μm). Smaller tannins and their gut metabolites only showed mild anti-SARS-CoV-2 Mpro effects.
CRCF Extension Laboratory (CEL) at Providence College
Kyle M. Medas, an undergraduate student from Dr. Seann P. Mulcahy’s lab, published his synthetic research work using Bruker Avance DRX 400MHz Nuclear Magnetic Resonance (NMR) spectrometer, located in the RI-INBRE’s CRCF extension laboratory (CEL) at Providence College. The researchers recorded proton and carbon NMR spectra to analyze intermediate and final products to develop the construction of elaborate pyridine-containing heterocycle. The scientists published their synthetic work in Organic Letters “Metal-Catalyzed Cyclotrimerization Reactions of Cyanamides: Synthesis of 2-Aryl-α-carbolines” in April 2020 (PMID32255636, PMC7895322).
Centralized Research Core Facility (CRCF) Extension Laboratories (CELs)
CELs are satellite laboratories that are an extension of the CRCF and located at RI-INBRE network institutions around the state of Rhode Island. CELs contain state-of-the-art instrumentation for the use and convenience of our investigators and students.
