Our current projects include studies of:
- microbial selection and community structure in subseafloor sediment,
- global distributions of subseafloor cells and activities,
- the energetics of subseafloor communities,
- ancient sedimentary (fossil) DNA
- pH variation in estuaries.
Many of our projects utilize the URI Geobiology Field Laboratory. All of them involve collaborations with talented scientists, including faculty, students, staff and postdocs at URI and other institutions around the world.
Current Projects
Microbial selection and community structure
We study microbial community structure and environmental selection in the sedimentand water column throughout much of the world ocean. Lab members Ramirez and Kerrigan, and lab alumni Kirkpatrick and Walsh, use high-throughput sequencing to document how bacterial and archaeal diversity and community composition change with environment and depth throughout the water column and sediment of the world ocean. For example, the deep chlorophyll maxima of different oceanic regions harbor distinct microbial communities that are consistent within regions for thousands of kilometers (Walsh et al., 2015, Aquatic Microbial Ecology). Our studies of community composition in the water column and sediment show that (i) sedimentary bacteria disperse via the ocean and (ii) the subseafloor sedimentary community is selected from the community present in near-seafloor sediment (Walsh et al., 2016, The ISME Journal). Bacterial diversity in anoxic subseafloor sediment declines exponentially with sediment age, in parallel with organic-fueled oxidation rate (Walsh et al., 2016, Applied and Environmental Microbiology). In anoxic marine sediment, it takes more than a hundred thousand years to decrease diversity from near-seafloor values to deep subseafloor values (Walsh et al., 2016, AEM).
Global distributions of subseafloor cells and activities
Our group has long been interested in global distributions of subseafloor habitability and life. Our first study of global subseafloor habitability and activity focused on global distributions of dissolved sulfate and methane in marine sediment, and some of their implications for sedimentary anaerobic communities (D’Hondt et al., 2002, Science). Principal results of that study are described in the Microbial energetics section below. Our most recent publication on global habitability and activity focused on the global distribution of dissolved oxygen in marine sediment, and some of its implications for sedimentary aerobic communities (D’Hondt et al., 2015, Nature Geoscience). In this study, we identified a relationship of O2 penetration depth to sedimentation rate and sediment thickness. Extrapolating this relationship, we suggested that oxygen and aerobic communities may occur throughout the entire sediment sequence in 15–44% of the Pacific and 9–37% of the global seafloor. Subduction of the sediment and basalt from these regions is a source of oxidized material to the mantle. On geologically long timescales, Earth’s surface oxidation state may be affected by the balance between material subducted from these relatively oxidized regions and reduced material subducted from regions with anaerobic subseafloor ecosystems. Much of the data used for our study of aerobic subseafloor communities study came from our field expeditions (D’Hondt et al., 2009, PNAS; Røy et al., 2012, Science; D’Hondt et al., 2015, Nature Geoscience).
Our global biomass study demonstrated that cell abundance and biomass in subseafloor sediment vary by orders of magnitude from one region of the world ocean to another, in concert with mean sedimentation rate and distance from shore (Kallmeyer et al., 2012, PNAS). Based on these correlations, we estimate global subseafloor sedimentary microbial abundance to be 2.9 x 1029 cells, corresponding to 4.1 petagrams of carbon (∼0.6% of Earth’s total living biomass). In short, there are roughly as many cells in marine sediment as in the overlying ocean. However, biomass is much lower in the sediment, because the cells in marine sediment are generally much smaller than those in the ocean. Much of the data used for this study came from our field expeditions (D’Hondt et al., 2004, Science; D’Hondt et al., 2009, PNAS; Kallmeyer et al., 2012, PNAS).
We are testing models for global distributions of subseafloor microbial activities. Our results will provide a firm basis for quantifying the impact of subseafloor activity on global biogeochemical cycles and predicting global patterns of microbial activities and energetic habitability in subseafloor sediment. As with our biomass study, the results to date demonstrate that organic-fueled respiration in subseafloor sediment varies by orders of magnitude from region to region, in concert with oceanographic properties. Much of the fundamental data used in this study also comes from our field expeditions (D’Hondt et al., 2004, Science; D’Hondt et al., 2009, PNAS; D’Hondt et al., 2011, Proceedings IODP; Wehrmann et al, 2011, Chemical Geology; D’Hondt et al., 2015, Nature Geoscience).
Limits to life
We are very interested in testing the physical, chemical, and energetic limits of life. Subseafloor gradients in environmental conditions and biological states occur over distances that permit highly resolved sampling.
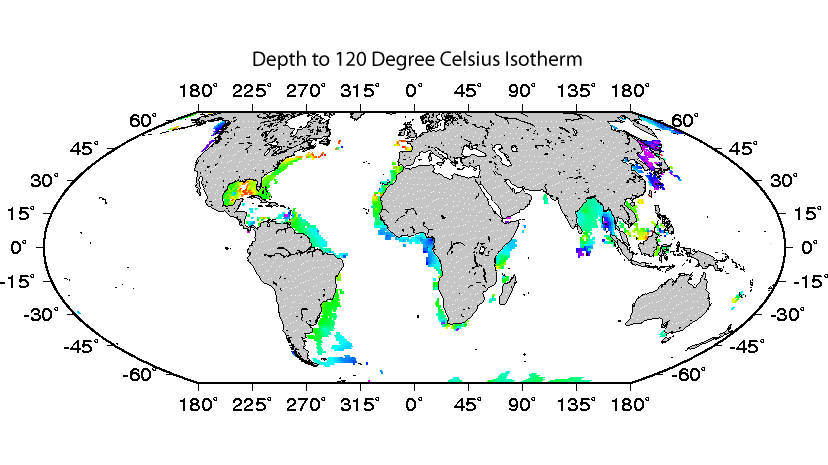
High temperature provides an obvious example of a limit to life and habitability that can be effectively studied through subseafloor exploration. A commonly accepted upper temperature limit for life is 122°C (Takai et al, 2008, PNAS). This temperature is reached in marine sediment at depths as shallow as 0 meters below seafloor and as great as kilometers below seafloor (click here for our subseafloor isotherm maps from D’Hondt and Rutherford, 2002, JOI/USSAC Newsletter).
Availability of energy (electron donors and electron acceptors) may also limit the occurrence of life. In some subseafloor environments, energy availability may be too low to maintain life. In others, life-sustaining fluxes of electron donors may be independent of photosynthesis and the surface world. In order to determine the energetic limits to life and the organisms that live on the energetic edge, these chemical constraints should be studied in a broad range of environments, including, but not limited to, sediment with very low organic content, deep crustal rocks, and subseafloor regions of active serpentinization.
We sampled and analyzed the entire sediment sequence and underlying basalt in the central South Pacific (IODP Expedition 329) to test the extent to which life is limited by very low organic content in sediment and the extent to which it is limited by very low availability of inorganic electron donors and/or electron acceptors in ancient (up to 120-million-year-old) subseafloor basalt. Our results show that there is no depth limit to life in this extremely organic-poor sediment – microbial cells and aerobic respiration persist through the entire sediment sequence (D’Hondt et al., 2015, Nature Geoscience). This discovery overturns the long-standing paradigm that the limit to life occurs just a few meters below the seafloor in Pacific abyssal clay (Morita and Zobell, 1955, Deep-Sea Research).
Microbial energetics
Our 2002 Science paper demonstrated that microbial communities in anoxic marine sediment are sustained by mean per-cell respiration rates that are up to six orders of magnitude lower than mean rates in microbial cultures and up three orders of magnitude lower than rates in seafloor sediment (D’Hondt et al., 2002, Science). Our recent studies have shown that per-cell respiration rates are similarly low in oxic subseafloor sediment (Røy et al., 2012, Science; D’Hondt et al., 2015, Nature Geosciences). Many subsequent studies have also reported that mean per-cell respiration rates are generally very low in subseafloor sediment (See reviews by Colwell & D’Hondt, 2013, Reviews in Mineralogy & Geochemistry; Orcutt et al., 2013, Frontiers in Microbiology, and Hoehler & Jørgensen, 2013, Nature Reviews: Microbiology). Although these rates are very low, Gibbs energy yields per subseafloor reaction are roughly equal to yields for the same reactions in surface environments (Wang et al., 2010, GCA). Combining in situ energies per reaction with per-cell respiration rates demonstrates that mean per-cell energy fluxes are also extraordinarily low in subseafloor sediment (D’Hondt et al., 2014, Developments in Marine Geology). Turnover times of microbial biomass in subseafloor sediment are correspondingly also very low (hundreds to thousands of years) (Lomstein et al., 2012, Nature). Comparison of these energy flux estimates and biomass turnover rates suggests that most of the energy flux to subseafloor sedimentary microbes may be used for building biomolecules from existing components, rather than for de novo biosynthesis (D’Hondt et al., 2014, Developments).
Despite these very slow rates of activity, deep subseafloor communities are metabolically complex. Potentially competing metabolic pathways co-occur for hundreds of meters in subseafloor sediment deposited over millions of years (D’Hondt et al., 2002, Science; D’Hondt et al., 2004, Science; Jørgensen et al., 2006, ODP Scientific Results 201). The activites of subseafloor sedimentary microbes appear to include previously unknown processes, such as biological production of ethane and propane (Hinrichs et al., 2006, PNAS) and sulfate-reducing ammonium oxidation (Schrum et al., 2009, Geology). Mutualistic interactions sustain these communities for millions of years with extremely little ongoing input of organic matter (Wang et al., 2010, GCA; D’Hondt et al., 2014; Developments).
Hydrogen from splitting of water by radioactivity may be a significant electron donor (food) in organic-poor marine sediment (Blair et al., 2007, Astrobiology; D’Hondt et al., 2009, PNAS) and the principal electron donor in deep continental hard-rock aquifers (Pedersen, 1997, FEMS Microbiol. Rev.; Lin et al., 2005, GCA) . The process of radiolytic hydrogen generation is even more effective in fine-grained marine sediment than in deep subsurface fractures (Blair et al., 2007).
Lab member Sauvage and lab alumnus Dzaugis are using samples and data from Integrated Ocean Drilling Program Expedition 329 and North Atlantic long-coring expedition KN-223 to (i) experimentally determine rates of radiolytic hydrogen production in seawater and marine sediment (Sauvage et al., in preparation), (ii) quantify rates and assess the biological importance of radiolytic hydrogen production in deep-sea sediment (Sauvage et al., in preparation), (iii) quantify rates and assess the potential biological importance of radiolytic hydrogen production in subseafloor basalt (Dzaugis et al., 2016, Frontiers in Microbiology), and (iv) quantify rates of radiolytic H2 production in water-saturated environments on Mars (e.g., the ancient Martian surface and the present Martian subsurface) (Dzaugis et al., in review).
To advance studies of radiolysis and life in fractured hard-rock systems, Dzaugis and coauthors developed a mathematical model for calculating water radiolysis rates near solid-fluid interfaces (Dzaugis et al., 2015, Radiation Physics and Chemistry). Model output includes dose-rate profiles, as well as production rates of H2 and H2O2. The model is useful for studies of both natural environments and human-made systems (such as storage reservoirs for spent nuclear fuel).
Ancient sedimentary (fossil) DNA
DNA in marine sediment contains both fossil sequences and sequences from organisms that live in the sediment. We are exploring ways to distinguish between these two pools, and to use them to respectively understand past and present states of the world. In our study of Bering Sea sediment, most fossil chloroplast DNA disappears within 100,000 to 200,000 of sediment deposition, but traces of silicious microfossil DNA persist to at least 1.4 million years (Kirkpatrick et al., 2016, Geology). Such persistent DNA may be useful for study of paleonvironmental conditions and biological evolution on timescales that approach or exceed one million years.
pH in estuaries
Acidification by anthropogenic CO2 may significantly impact estuaries, which are environments of particular biological and human importance. We have assessed this impact by (i) examining pH in three geographically distinct estuaries (D’Hondt et al., in preparation) and (ii) measuring hourly variation in pH, alkalinity and dissolved inorganic carbon over representative days throughout the year at three Narragansett Bay sites (Turner, 2015 URI M.S. thesis; Turner et al., in preparation). In each estuary, pH varies by up to 1.5 units over a year and one unit over a day. The mean pH of each is well below that of the surface open ocean. Comparison to culturing studies indicates that mean pH in western Atlantic estuaries may already hinder larval survival and growth of some organisms (Talmage and C.J. Gobler, 2009, Limnol. Oceanogr.; Miller et al, 2009, PLoS ONE). Estuarine pH variation is predictable on annual and diel/subdiel timescales. This variation is primarily driven by photosynthesis and respiration. If these biological processes operate similarly in the future, as atmospheric CO2 rises, estuarine ecosystems will be chronically subject to far lower pH than open ocean ecosystems (D’Hondt et al., in preparation).